Abstract
Background:
Outcomes of patients with relapsed/refractory AML (RR-AML) have remained poor. Therapy with venetoclax based combinations in this setting leads to CR/CRi rates of 21-49% (DiNardo CD et al. Am J Hematol 2018, Aldoss I et al. Haematologica 2018, Stahl M et al. Blood Adv 2020). Preclinical studies with BCL-2 inhibitors indicate potential mechanisms of drug resistance including overexpression of the anti-apoptotic protein MCL-1 (Konopleva et al. Cancer Cell. 2006). Pevonedistat is a first in class inhibitor of Nedd8 activating enzyme that induces the pro-apoptotic protein NOXA leading to neutralization of MCL-1 and apoptosis. Preclinical studies evaluating the combination of pevonedistat and venetoclax against AML cell lines have demonstrated a synergistic effect (Knorr KL et al. Cell Death Differ. 2015). Hence, we designed a phase I study to assess the safety and tolerability of adding pevonedistat to the combination of azacitidine and venetoclax in patients with RR-AML.
Study design and methods:
We conducted a phase I study with the combination of pevonedistat, venetoclax and azacitidine in patients with RR-AML. Patients aged 18 years or above with morphologically documented RR-AML, ECOG performance status 0-2 and adequate organ function were eligible. Major exclusion criteria were isolated extramedullary relapse, hematopoietic cell transplantation (HCT) within 100 days of enrollment, and active acute GVHD. Previous therapy with hypomethylating agents (HMA) or venetoclax was not an exclusion criterion. The dose escalation phase was conducted using 3+3 design. Treatment included azacitidine (75 mg/m 2 daily x 7 days), venetoclax (400 mg daily x 28 days), and pevonedistat in escalating doses (10-20 mg/m 2 IV days 1,3,5 of each cycle) with a cycle length of 28 days. Pevonedistat was given at 10 mg/m 2 dose in cohort 1, 15 mg/m 2 dose in cohort 2 and 20 mg/m 2 dose in cohort 3.
The primary endpoint is to determine the recommended phase 2 dose (RP2D) and toxicity profile of pevonedistat, azacitidine and venetoclax. Other endpoints included determination of response rates, duration of response, survival, pharmacokinetics, correlation of response rates with AML genomic profile, correlation of pretreatment levels of BCL2, BCLXL, MCL1, BAX or BAK with response, determination of changes in NOXA (PMAIP1) mRNA and protein expression pre-and post-pevonedistat treatment, evaluation of BH3 mimetic profiling on bone marrow samples by flow cytometry and assessing the sensitivity of leukemia and leukemic stem/progenitor cells to pevonedistat ex vivo.
Results:
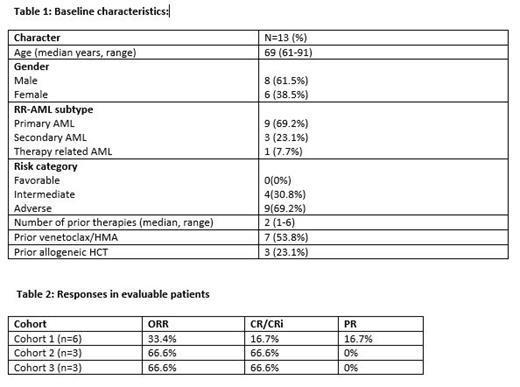
Thirteen patients participated in the dose escalation phase, 12 of whom were evaluable. Median age was 69 years (61-91), 30.8% had secondary/therapy related AML, 69.2% with adverse risk disease, 53.8% previously received venetoclax/HMA and 23.1% had relapse after prior allogeneic hematopoietic cell transplantation (HCT) (Table 1). Seven patients were enrolled into cohort 1 (pevonedistat 10 mg/m 2 dose) of which one was not evaluable, three patients enrolled into cohort 2 (pevonedistat 15 mg/m 2 dose), and three patients enrolled into cohort 3 (pevonedistat 20 mg/m 2 dose). Grade 3 or higher AEs included febrile neutropenia (23%), infection (15%), anemia (38%), neutropenia (54%), thrombocytopenia (38%). There was 1 dose limiting toxicity (DLT) in cohort 1 (atrial fibrillation) that triggered cohort expansion. However, subsequent patients did not experience DLT despite planned dose escalation. Of the 12 evaluable total patients, CR/CRi was observed in 5 (41.6%) patients. Notably, patients with RR-AML who were venetoclax/HMA naïve had a CR/CRi 83.3% (5/6 patients). The response rates for each cohort are summarized in Table 2. Three of the five patients with CR/CRi (60%) achieved MRD negativity by flow cytometry. Four patients who achieved CR/CRi proceeded to allogeneic HCT after therapy. Median OS of the cohort was 5.4 months (1.8-14) and median OS was not reached in patients with CR/CRi.
Conclusions:
The addition of pevonedistat to venetoclax and azacitidine backbone is safe and well tolerated in patients with RR-AML. Dose escalation yielded encouraging efficacy in venetoclax/HMA naïve RR-AML patients. The study is currently in the dose expansion phase. Details on correlative studies examining mechanisms of therapeutic efficacy and resistance will be reported in the main meeting.
Guru Murthy: Cardinal health: Honoraria; TG Therapeutics: Other: Advisory board meeting; DAVA Oncology: Honoraria; CancerExpertNow: Honoraria; Qessential: Honoraria; Techspert: Consultancy; Curio Sciences: Honoraria; Guidepoint: Consultancy. Abedin: AltruBio: Research Funding; Helsinn: Research Funding; Amgen: Honoraria; Pfizer: Research Funding; Agios: Honoraria; Actinium: Research Funding; Astellas Pharma Inc.: Research Funding. Litzow: Jazz: Other: Advisory Board; Pluristem: Research Funding; Actinium: Research Funding; Amgen: Research Funding; Astellas: Research Funding; AbbVie: Research Funding; Omeros: Other: Advisory Board; Biosight: Other: Data monitoring committee. Atallah: Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Research Funding; BMS: Honoraria, Speakers Bureau; Takeda: Consultancy, Research Funding; Abbvie: Consultancy, Speakers Bureau; Amgen: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal